We all know that fossils fuels are playing important role in the development of any country economically and technically. Every country needs a power or energy source for its proper functioning and operation. For this, we all are dependent on fossils energy sources. But there is a limitation in the use of these fuels. Fossil fuels consumptions are higher than their production rate and it emits harmful gases those are making toxic environment for human beings.
To handle these problems human beings are continuously trying to find out other energy sources and Fuel Cells is one of them. So, without any further delay, it’s time to explore the technology behind the invention.
What is a Fuel Cell?
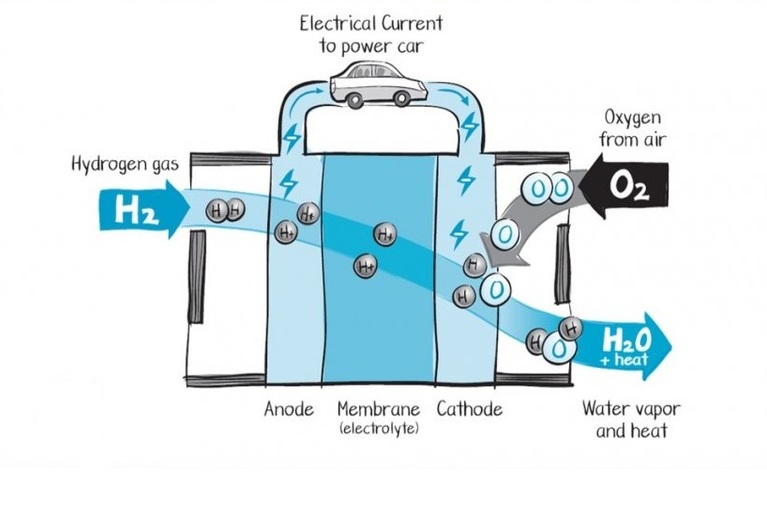
A fuel cell is a kind of electrical generator that generally uses hydrogen as a fuel. The fuel cell uses the chemical energy of hydrogen or other fuels to generate clean and efficient energy. If hydrogen is being used inside a fuel cell then the products are electricity, water, and heat only.
Fuel cells have several benefits over conventional energy sources. These cells can operate at higher efficiencies than combustion engines and can convert the chemical energy in the fuel directly to electrical energy with an efficiency of up to 60%. Fuel cells have zero or very few emissions compared to conventional combustion engines. Hydrogen-based fuels cells emit only water as waste and there are no carbon emissions. And also, there are no air pollutants that cause health issues for living organisms on earth. Fuel cells are also known as silent power generators because it has few moving parts during its operation.
Working Process:
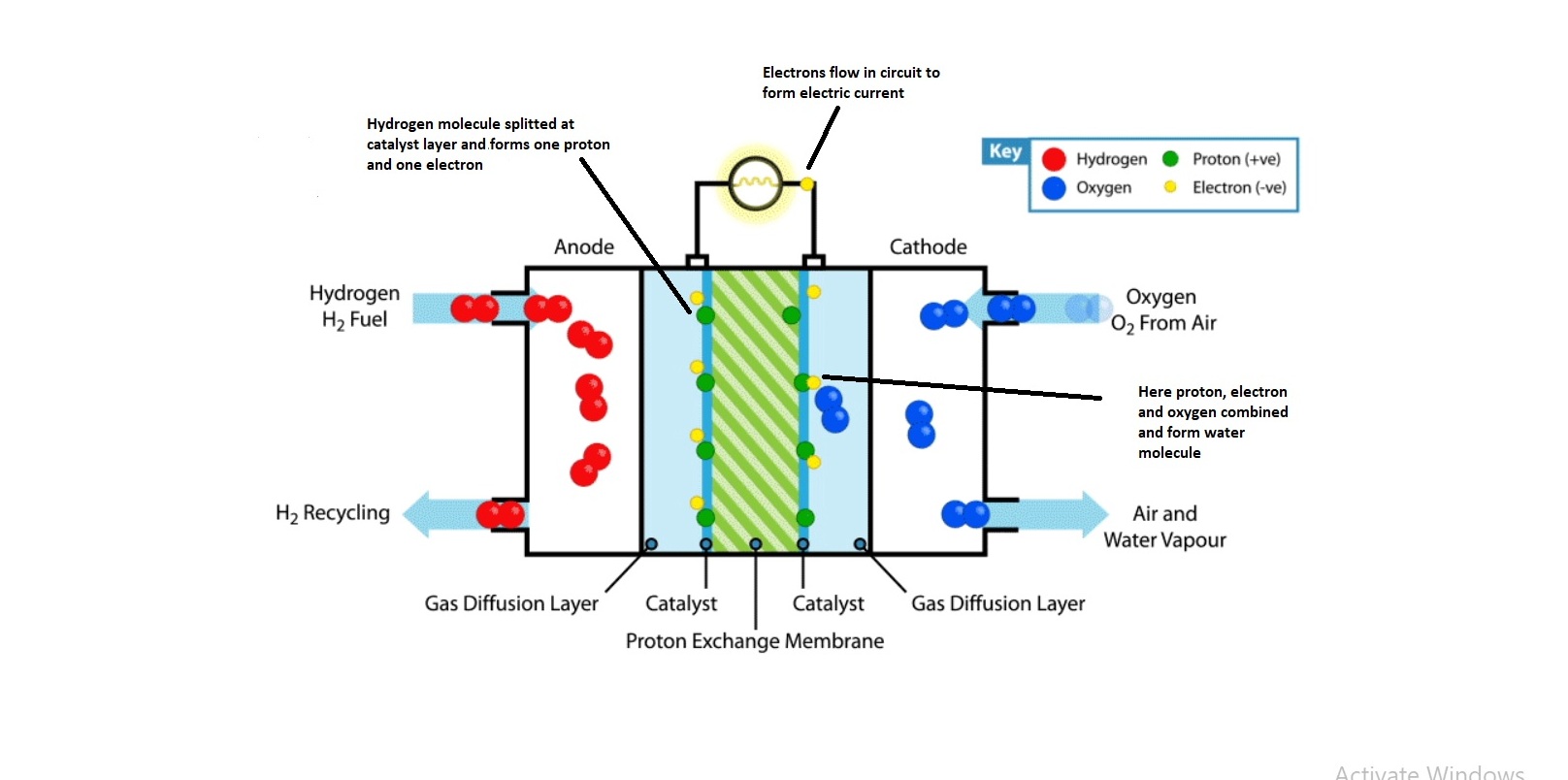
Fuel cells are similar to batteries, but they do not need recharging. They produce electricity and heat as long as fuel (hydrogen) is supplied. A fuel cell contains two electrodes – the first one is Anode (known as a negative electrode) and the second one is a cathode (positive electrode). These electrodes are sandwiched around an electrolyte. Fuel like hydrogen is fed to the anode, and the air is fed to the cathode. In a hydrogen fuel cell, a catalyst (thin layer of platinum) at the anode separates hydrogen molecules into protons and electrons. These changes take different paths to the cathode. The electrons go through an external circuit, creating a flow of electric current whereas protons migrate through the electrolyte to the cathode. At the cathode, protons unite with electrons, oxygen and produce water and heat.
Parts of Fuel Cell:
Fuel cell contains synthetic polymer membrane. Polymer electrolyte membrane (PEM) type fuel cells are popular nowadays. PEM fuel cells are made from several layers of different materials. Some important parts of the PEM fuel cell are given below:
Membrane Electrode Assembly (MEA):
The heart of a PEM fuel cell is the membrane electrode assembly (MEA). It includes the polymer electrolyte membrane (PEM), the catalyst layers, and gas diffusion layers (GDLs).
Polymer Electrolyte Membrane:
The polymer electrolyte membrane is a specially treated material that looks something like ordinary kitchen plastic wrap. This electrolyte conducts only positive charges (protons) and blocks the electrons. The PEM is the key to fuel cell technology. Other materials passing through the electrolyte would disrupt the chemical reaction of the fuel cell.
Working process of fuel cell
Catalyst Layers:
A layer of catalyst is added on both sides of the polymer electrolyte membrane. Conventional catalyst layers include nanometer-sized particles of platinum dispersed on a high surface area carbon support. This supported catalyst is mixed with an ion-conducting polymer and also sandwiched between PEM and gas diffusion layers (GDLs). On the anode side, the catalyst (generally platinum) enables hydrogen molecules to break into protons and electrons. At the cathode end, the catalyst enables oxygen reduction by reacting with the protons that were generated by the anode. Protons generate water at the cathode end.
Gas Diffusion Layers:
The gas diffusion layers present outside the catalyst layers facilitate the transport of reactants gases H2, Air/Oxygen, and product gases. Each gas transport layer is typically composed of a sheet of carbon paper in which the carbon fibers are partially coated with “Poly Tetra Fluoro Ethylene” (PTFE). PTFE is also commonly known as Teflon. The Teflon layer prevents excessive water buildup because the water buildup may block the pores of GDL paper. In many cases, the inner surface of the GDL is coated with a thin layer of high-surface-area carbon mixed Teflon, called the micro-porous layer. This micro-porous layer can help to adjust the balance between water retention (water required to maintain membrane conductivity) and water release (needed to keep the pores open so hydrogen and oxygen can diffuse into the fuel cell via electrodes.
Basic Components in a Fuel Cell:
Fuel Cell Stack:
The fuel cell stack in this energy system generates electricity in the form of DC or direct current from the electro-chemical reaction. A single fuel cell produces approximately 1V, which is not sufficient to operate any electrical equipment. Therefore, individual fuel cells are combined to form a stack of the fuel cell. Typically a fuel cell stack may contain more than hundreds of fuel cells.
Fuel Processor:
The fuel processor converts fuel into a form usable by the fuel cell. Primarily, a fuel processor is a type of chemical reactor that converts a commonly available fuel, such as gasoline, methanol, or natural gas, into a gas stream containing primarily the compound(s) required by the fuel cell such as hydrogen (H2) or methane (CH4).
Power Conditioners:
Power conditioning include controlling voltage, current, frequency, and another important characteristic of the electrical current to meet the needs of the application. Fuel cells generate direct current. Here power conditioners use to handle the conditioning of direct current.
Applications:
Fuel cells are unique in terms of the variety of their potential applications. They can use a wide range of fuels and feedstocks and can produce power for the system as large as a utility power station and small as a computer system. In the upcoming days improved model of the fuel cell will replace household inverters for power generation. Fuel cells are also being used with superconducting coils in space sectors.
Thanks for reading. See you soon with another exploration!




[…] electricity to perform a specific task. As of now, we are using batteries, solar power, wind power, fuel cells, and more. We have seen various methods to generate electricity either by using a chemical reaction […]